Nano-mechanics of phagocytosis and filopodia
Background: Phagocytosis is a central mechanism in our immune system used by cells, e.g. macrophages, to take up bacteria and other particles. This uptake can decide on life or death of a cell or a whole organism. Problem: The interaction of the cytoskeleton and the molecular motors driving the uptake by the cell is not fully understood. Despite large biochemical knowledge, the origin of the forces and the fluctuating energies leading to the uptake by molecular self-organization need to be understood in much more detail. Approach: We use 3D optical trapping and tracking of one micron sized polystyrene particles and approach them to the cell periphery. We drive different perturbations patterns to induce various responses of the cell to take up the particle. Temporal and spatial analysis of the particle fluctuations at 1 MHz reveal biophysical processes on a molecular scale. | 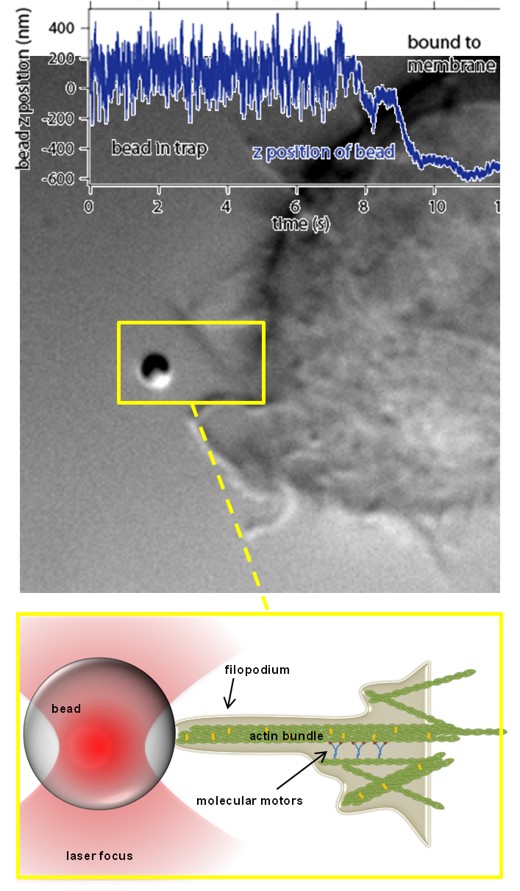 |
Particle-cell interaction on a thermal noise level
Among all the external perturbations that a cell is consistently exposed to, mechanical interactions are of paramount importance in physics, biology, medicine and pharmacy. To understand the statistical mechanical principles that govern cellular reactions to an approaching particle, it is necessary to understand its thermal motion on different timescales. While interactions can be visible on one timescale, they can be completely invisible on another.
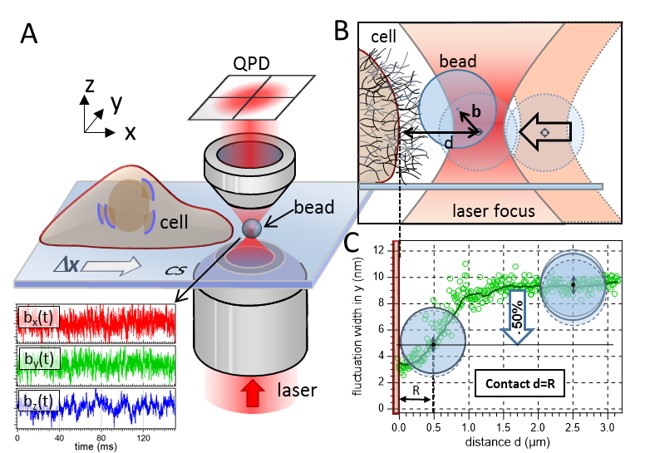
(A) Sketch of the experimental setup. A 1 µm bead is fluctuating in the proximity of a living cell. An optical trap confines the motion of the particle to a small volume around the trap center. The trap is moved toward the cell membrane with Δy = 20 nm steps by means of a 3D piezo stage. The bead’s fluctuation data are recorded in three dimensions (lower part of A) with 2 MHz temporal resolution via two quadrant photo diodes (QPDs). (B) The distance between the center of the optical trap and the cell membrane is denoted as d, whereas b is the bead’s displacement from the trap center. The thin mesh-like strands on top the cell membrane illustrate the cell’s ECM. (C) The contact point between cell membrane and bead surface is determined by a 50% decrease of the bead’s radial fluctuation width. |
Nano-mechanics of filopodia
| Confocal time series of dorsal filopodia of J774 macrophage cells with labelled actin cytoskeleton. Maximum intensity z-projection of a 3D stack. The surface of the cells is structured by many actin filled protrusions and filopodia. | 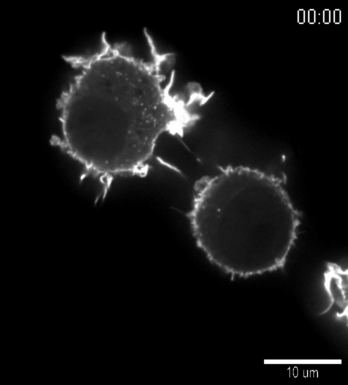 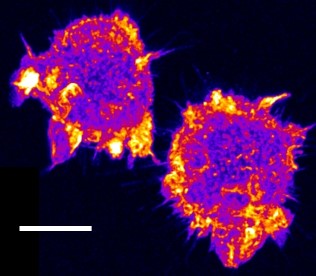 |
Adaptive filopodia
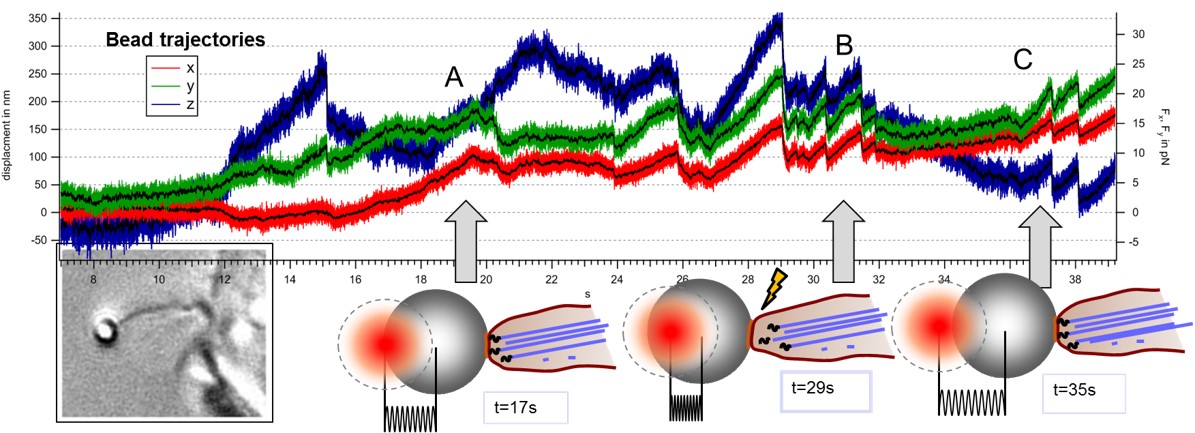
Modes of transport observed in bead trajectories, indicating that stability of actin-integrin-membrane bonds is increased stepwise by the cell to overcome high counteracting forces.
A: directed pulling of filopodium with actin backbone attached
B: failure of connection between actin and membrane, bead relaxes back in trap
C: after breakage filopodium reconnects and resumes pulling several times until the filopodium is finally succesful
Modes of transport observed in bead trajectories, indicating that stability of actin-integrin-membrane bonds is increased stepwise by the cell to overcome high counteracting forces.
A: directed pulling of filopodium with actin backbone attached
B: failure of connection between actin and membrane, bead relaxes back in trap
C: after breakage filopodium reconnects and resumes pulling several times until the filopodium is finally succesful
Strong cytoskeleton activity on millisecond timescales during particle binding and uptake
Activity analysis of a J774 macrophage handling 350nm glass beads, obtained by an image sequence with fs = 44 Hz.
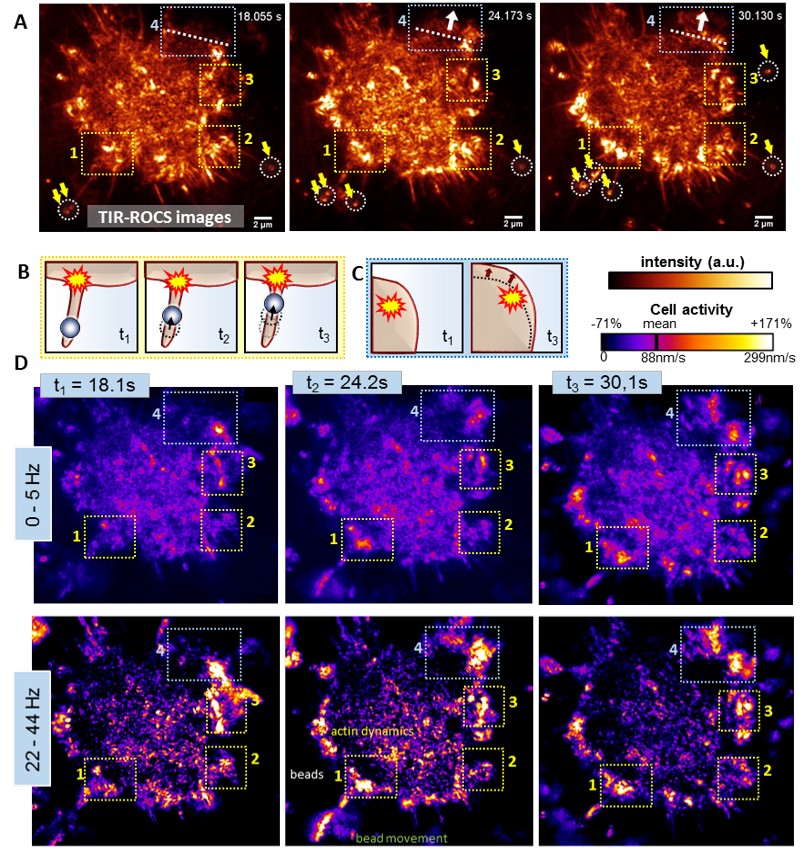
(A) Darkfield TIR-ROCS images of the cell at 3 different time points with beads marked by white circles and yellow arrows, revealing two observed processes, which are sketched in (B,C): (B) Bead transport along an adherent filopodium and (C) cell cortex movement in north-eastern direction (indicated by white dotted lines and arrows in A). (D) Corresponding frequency-resolved activity maps for two frequency ranges (0-5Hz and 22-44Hz) as indicated on the left. Yellow boxes ‘1’-‘3’ highlight regions, in which the uptake of several beads via adherent filopodia is initiated (sketch (B)) and with significantly increased activities on short timescales. The light blue box ‘4’ highlights an area associated with cortex movements, (sketch (C)).
Publications
- Jünger F, Rohrbach A
Strong cytoskeleton activity on millisecond timescales during particle binding and uptake revealed by ROCS microscopy
2018 Cytoskeleton, Volume: 75, pages: 410 - 424 - Jünger F, Kohler F, Meinel A, Meyer T, Nitschke R, Erhard B, Rohrbach A
Measuring local viscosities near plasma membranes of living cells with photonic force microscopy
2015 Biophys J, Volume: 109, Nummer: 5, pages: 869 - 882 - Kohler F, Rohrbach A
Surfing along filopodia – a particle transport revealed by molecular scale fluctuation analyses
2015 Biophys J, Volume: 108, pages: 2114 - 2125
- H. Kress, E. H. K. Stelzer, D. Holzer, F. Buss, G. Griffiths, A. Rohrbach
Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity 2007 Proc. Nat. Acad. Sci., Volume: 104, pages: 11633 - 11638